kimberly clark clo test package insert|clo test procedure pdf : services The Avanos Medical CLOTEST* Jack Bean Urease Control Tablets are the positive control for CLOTEST* Rapid Urease Test, one of the most highly regarded urease tests available to medical professionals. Its combination of . Pour vous identifier, entrez le code à 8 chiffres qui apparaît sur votre TV ou votre appareil connecté
{plog:ftitle_list}
Abaixo você encontra tabelas com os melhores jogadores do dia no e-soccer de 10, 12 e 8 minutos (Adriatic League antes Live Arena, GT Leagues e Esoccer Battle) da Bet365 com base somente nos jogos de hoje (375) para apostas esportivas em FIFA. * Atualizado a cada 3 minutos (28/02/2024)
clo test procedure pdf
The CLOtest* Rapid Urease Test is used for the diagnosis of H. pylori. The Bacteriostatic agent in the gel decreases the likelihood of false positives, and .The CLOtest rapid urease test accurately and conveniently detects the urease enzyme of Helicobacter pylori in gastric mucosal biopsies. Its use is intended for the presumptive . These tests included culture, histology, and the rapid urease test (CLO test). Methods. H. pylori infection was diagnosed prospectively in 246 untreated dyspeptic patients who underwent upper gastrointestinal endoscopy. The gold standard for H. pylori infection was based on a positive culture or both a positive histological examination and a .The Avanos Medical CLOTEST* Jack Bean Urease Control Tablets are the positive control for CLOTEST* Rapid Urease Test, one of the most highly regarded urease tests available to medical professionals. Its combination of .
Test Panels Package Insert Adulteration Color Chart (when applicable) Materials Required But Not Provided Timer Specimen collection containers 【DIRECTIONS FOR USE】 Allow the test, urine specimen, and/or controls to reach .Solutions. Digestive Health Solutions. CORTRAK* 2 Enteral Access System (EAS) Enteral Feeding Supplies & Products. MIC-KEY* Feeding Tube Kits; Percutaneous Endoscopic Gastrostomy (PEG) Tubes
sealey compression test kit
By Sue Reisinger. Law360 (February 26, 2024, 4:19 PM EST) -- The former chief legal and business development officer of Kimberly-Clark Corp. earned over .2 million in compensation in 2023 . Background: Rapid diagnostic tools for Helicobacter pylori are important in endoscopy. Aims: To assess the accuracy of a new 5 min rapid urease test (UFT300, ABS Srl, Cernusco sul Naviglio, Milan, Italy) and to compare it with the 1 h Pyloritek (Serim Laboratories, Elkhart, IN, USA) and the 24 h CLO test (Kimberly-Clark Ballard Medical Products, Roswell, .1. Open the package containing the C-urea capsule minute and tip the capsule into the empty 30 mL cup. Do not handle the capsule directly. 2. Hand the cup to the patient. The kit includes analysis by Kimberly-Clark of one balloon from one patient at one time point. 1 14BUDDY PASS NOW AVAILABLE on CLO Symposium Registration, CLO Accelerator Enrollment and Membership. . About Kimberly-Clark. Kimberly-Clark’s Learning & Development Center of Excellence leveraged technology to reach more learners through a global LMS. The goals for the shift were to track compliance training and provide global learning .

Background Rapid diagnostic tools for Helicobacter pylori are important in endoscopy.. Aims To assess the accuracy of a new 5 min rapid urease test (UFT300, ABS Srl, Cernusco sul Naviglio, Milan, Italy) and to compare it with the 1 h Pyloritek (Serim Laboratories, Elkhart, IN, USA) and the 24 h CLO test (Kimberly-Clark Ballard Medical Products, Roswell, . H. pylori urease 2 13C-urea + 2 H 2O 2 → 213CO 2 + 2 NH 3.The 13CO 2 is absorbed into the blood and then exhaled in the breath. Absorption and distribution of 13CO2 is fast. Therefore, the cleavage of urea by the H. pylori urease that produces the 13CO 2 occurs immediately after the solution is ingested and enables immediate detection of increased 13CO
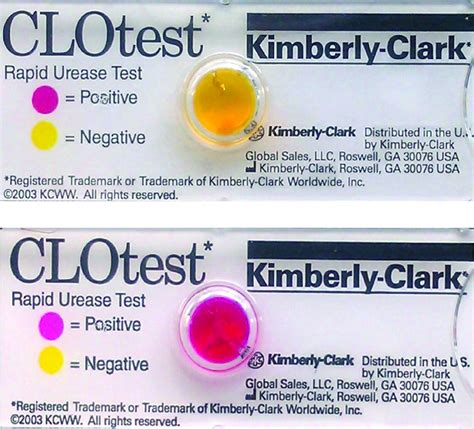
우리나라에서는 CLOtest, ultrarapid urease test, urease reagent strip test등이 사용되고 있다. 요소분해효소 검사의 예민도는 85-97%, 특이도는 약 92%로, 예민도와 특이도가 모두 높아 내시경 검사가 가능한 의료기관에서 헬리코박터 감염의 진단에 있어 1차 .The Multi-Drug Rapid Test Panel is a rapid urine screening test that can be performed without the use of an instrument. The test utilizes monoclonal antibodies to selectively detect elevated levels of specific drugs in urine. Acetaminophen (ACE) Acetaminophen is one of the most commonly used drugs, yet it is also an important cause of serious liverCLOtest* will diagnose 75% of H. Pylori infections with no false positives within 20 minutes4. Within one hour, 85% of positive patients will be detected by CLOtest, at 3 hours, 90% are detected, and between 3 and 24 hours another 5% of patients will be detected by CLOtest*. 2 Reaction Time CLOtest* Rapid Urease Test Product Code Description .P Y T e s t TM package insert. Ballard Medical Products, Draper, Utah; August 1997. Soll AH. Consensus Statement. Medical treatment of peptic ulcer disease - practice guidelines. J A M A. 1996;275:622–629. Stubbs JB, Marshall BJ. Radiation dose estimates for the C-14 labeled urea breath test. J Nucl Med. 1993;34:821–825. VIII. Disclaimer
152 Years in Business Since 1872, Kimberly-Clark’s 44,000 employees around the world are. Keep Reading
Explore Kimberly-Clark. From Sales, Brand Management and Marketing to Finance,Technology, Manufacturing, Supply Chain, Research & Engineering and more – Kimberly-Clark abounds with opportunities for you to explore. Please note: We embrace the technology advances that result in far greater flexibility in our communications and business operations.
How many tissues should the Kimberly Clark Corporation package of Kleenex ® contain? Researchers determined that 60 tissues is the mean number of tissues used during a cold. Suppose a random sample of 100 Kleenex users yielded the following data on the number of tissues used during a cold: = 52, S = 22. At endoscopy two sets of biopsies, taken in random order, were placed in the wells of the Campylobacter-like organism (CLO) test (Kimberly-Clark, Utah, USA) and the Quick test (Biohit Plc, Helsinki, Finland). Five additional gastric biopsies were taken for histology/Giemsa and immunohistochemical study. The two urease test slides were read at 2 .How many Kleenex should the Kimberly Clark Corporation package of tissue contain? Researchers determined that 60 tissues is the average number of tissues used during a cold. Suppose a random sample of 100 Kleenex users yielded the following data on the number of tissues used during a cold: . Since the critical test value is 1.96, we have .
clo test for h pylori
About Kimberly Clo. I am a visual artist who is ruthlessly devoted to communicating LOVE through ordinary means and the irresistible language of color, geometric expression, precious metals and a jillion dots. Connect with Kimberly on Social Media :: Instagram: @KimberlyClo.professional judgment should be applied to any drug of abuse test result, particularly when preliminary positive results are indicated. 【SUMMARY】 The Multi-Drug Rapid Test Panel is a rapid urine screening test that can be performed without the use of an instrument. The test utilizes monoclonal
The Multi-Drug Rapid Test Panel is releasea rapid urine screening test that can be performed without the use of an instrument. Oberlender,The test utilizes monoclonal antibodies to selectively detect elevated levels of specific drugs in urine. dose Acetaminophen (ACE)60494 PYtest* Validation Package, 1 Each 3- Collection Patient support website:Balloons, 3- Straws, 3- Results Forms, 3- Return Cartons KIMBERLY-CLARK* PYtest* 14C-Urea Breath Test Start-Up Package Provides supplies (except capsules and liquid scintillation counter equipment) to perform 100 tests on site.10 questions about Drug Test at Kimberly-Clark. When you get a promotion do you have to be drug tested again? Asked May 9, 2024. No, only done at initial hire. Answered May 9, 2024. Answer See 1 answer. Report. Do they drug test on the . How many Kleenex should Kimberly clark corporation package of tissues contain? researchers determined that 60 tissues is the average number of tissues during a cold. supposed the random sample of 10,000 Kleenex users yielded the following data on the number of tissues used during a cold: X-bar = 52, s = 22. Suppose the test
For CLO test (Kimberly‐Clark, Medex Supply, USA), 2‐3 mm specimen was placed into medium containing urea and was examined by the test following manufacturer's instructions. The CLO test results were evaluated 2 to 12 h after by endoscopy. The evaluation of the modified Giemsa stain and CLO test was performed at the Departmentof Pathology inRapid diagnostic tools for Helicobacter pylori are important in endoscopy. To assess the accuracy of a new 5-min rapid urease test (UFT300, ABS Cernusco, sul Naviglio, Italy) compared with the 1-h Pyloritek (Serim Laboratories, Elkhart, IN) and the 24-h CLO test (Kimberly-Clark Ballard Medical Products, Roswell, GA), consecutive dyspeptic patients referred to our unit for .
bjc clo test positive
bjc clo test guidelines
web25/01/2024. IMAGENS FORTES! CRUELDADE! Vídeo mostra momento em que jovem é executado a facadas. TUDO FILMADO. VEJA. Foto: Reprodução / PORTAL DO .
kimberly clark clo test package insert|clo test procedure pdf